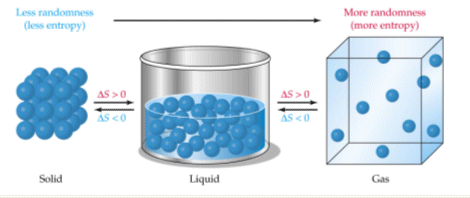
What most determines the entropy of a solid. On the other hand randomness and disorder decrease as a substance goes from gas to liquid and finally to solid.
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic
Correct option is A A.

. Which of the following reactions would have a decrease in entropy. Add your answer and earn points. - 20083531 angiepl53ap angiepl53ap 12142020 Chemistry.
2509 2802g O29 C. So an example of a reaction that leads to a decrease in entropy is. So right from the start you know that any reaction that has a gas as a reactant and a liquid as a product for example will result in a decrease in entropy.
Which of the following would result in a decrease in entropy. Hence in this reaction. Which of the following reactions would result in increased entropy.
2SO2 g O2 g yields 2SO3 g 2NH3 g yields N2 g 3H2 g 2H2O2 aq yields 2H2O l O2 g NaHCO3 aq HCl aq yields NaCl aq H2O l CO2 g 2. While other options reactants and products are in same phase gas phase having same entropy. Which of the following types of reactions would decrease the entropy of a cell.
The Reactions in which Δn is negative there will be a decrease in Entropyin the 1st reactionC s2H 2 g C H 4Δn No of moles of gaseous products - No of moles of gaseous reactantsΔn 1 - 2 -1Therefore there will be a decrease in entropy. Which of the following reactions results in a decrease in entropy. Which of the following reactions results in a decrease in entropy.
Δ H v e. Because recatants are in solid form having less enetropy and products are liquid form having more entropy. This is precipitation reaction in which the ions present in solution were in aqueous solution and have more freedom to move here and there so these had more entropy but when these forms the AgClthen these precipitate and the randomness is decreased as these are precipitated in the form of solid.
Which of the following reactions would result in decreased entropy. The system has become more random. CaCO 3 s CaO s CO 2 g N 2 O 4 g 2NO 2 g CaO s SO 3 g CaSO 4 s 2NH 3 g N 2 g 3H 2 g.
A chemical reaction that has a positive delta G is correctly described as endergonic. Indicate which one of the following reactions most certainly does not result in a decrease in entropy. OTICI4 1 TiCl4 g OPC13 g C12 g PC15 8 CO2 s CO2 g O2 NH3 g N2 g 3 H2 8 O2 NO3 g 2 NO2 g O2 g Question 2 4 points Consider 75 mL of a 0055 M ethylamine solution that is being titrated with 0200 M HCI.
Then choose the correct statements from the following a The process is spontaneous at all temperatures b The process is accompanied by an increase in entropy c The process is accompanied by a decrease in entropy d The process is accompanied by a decrease in enthalpy. What factors generally determine whether a reaction happens or not. In your case you have CH_ 3OH_ l - CO_ g 2H_ 2g.
2NH3g N2g 3H29. 203 9 302 9 O D. Chemistry questions and answers.
The crystalline structure of the solid. N2204 9 2NO39 maxcossagolia2004 is waiting for your help. AO2gO2l bCa2aq2ClaqCaCl2s cMgSO4s7H2OgMgSO47H2Os dNal12Cl2gNaCls e2NH3gN2g3H2g.
The types of reaction that would decrease the entropy within a cell is dehydration reactions. NH4Clsolid So you see there are more gaseous molecules in the reactant side of the equation. H20 g H20 1 O B.
For the reaction I 2 g I 2 s. Question 1 4 points Which of the following reactions will result in a decrease in entropy. A chemical reaction that has a positive delta G is correctly described as endergonic.
What does S 0 mean. C Every energy transfer requires activation energy from the environment. B If there is an increase in the energy of a system there must be a corresponding decrease in the energy of the rest of the universe.
The types of reaction that would decrease the entropy within a cell is dehydration reactions. Which of the following reactions would result in decrease entropy. 2H2Og 2H2g O29 B.
A If the entropy of a system increases there must be a corresponding decrease in the entropy of the universe. CO2 s CO2 9 O C.

Which Of The Following Reaction Would Result In A Decrease In Entropy Brainly Com

Chemical Thermodynamics Ppt Download

Which Of The Following Reactions Would Result In Decreased Entropy Multiple Choice Brainly Com
0 Comments